Automotive Material UNIT-1 (3 Marks)
3 marks Questions:
1. Explain the working principle of magnetic particle inspection testing.
A. MAGNETIC PARTICLE INSPECTION
Magnetic particle method is used for locating surface and subsurface dis- continuities in Ferro-magnetic materials. It is based on the fact that. when the material or the part under test is magnetized, magnetic discontinuities that lie in a direction generally transverse to the direction of the magnetic field, will cause a leakage field to be formed at and above the surface of the part. The presence of this leakage field, and therefore the presence of the discontinuity, is detected by the use of finely divided Ferro-magnetic part1- clues applied over the surface, some of the particles being gathered and held by the leakage field. This magnetically held collection of particles forms an outline of the discontinuity and generally indicates its location, size, shape and extent. Magnetic particles are applied over a surface as dry particles, or as wet particles in a liquid carrier such as water or oil.
One of the basic requirements of magnetic particle inspection is that the part undergoing inspection must be properly magnetized, so that leakage fields created by discontinuities will attract the magnetic particles. Ferro- magnetic materials can be magnetized in two different ways, either, by the use of permanent magnets, or by passing an electric current through the material.
A horse shoe magnet can be used to magnetize the material to be tested. The principle of working of a horse shoe magnet is explained in the Fig..When a magnetic material is placed across the poles of a horse shoe magnet having square ends, forming a closed or ring-like assembly,
the magnetic lines of force flow from the north pole through the magnetic material to the south pole and are entirely enclosed Within the ring-like assembly, because no external poles exist. As a result. any iron fillings or magnetic particles dusted over this assembly are not attracted to the magnet. But if one end of the magnet is not square, an air gap exists between that end of the magnet and the magnetic material. The poles (of the shoe-magnet) will than attract magnetic materials and the magnetic particles will cling-to the poles and bridge the gap between them. Similarly any radial crack in a circularly magnetized piece will create a north and a south magnetic pole at the edges of a crack. Magnetic particles will be attracted to the poles created by such a crack, giving an indication of the discontinuity in the piece.
When the magnetic flux is produced in a ferromagnetic material by pas- sing an electric current through a coil of wire wound around the material, the method is called Iiiagra flux inspection. Production of leakage field at the crack in a cylindrical bar by passing current through a solenoid coil is shown in Fig(b).
2. What is crystal imperfection? Enlist the types of imperfection in crystal structure?
A.CRYSTAL IMPERFFCTION
Real crystals deviate from the perfect periodicity of atoms Which is assumed in an ideal crystal. This deviation is chiefly responsible for the changes in the mechanical and electrical properties of the real crystals. This deviation of atoms from an orderly array of lattice points is termed as defect or imperfection. An understanding of these lattice defects is very important to explain the mechanical behaviour of metals. For example the actual strength- th of polycrystalline material is about 10 3 to 10 5 times lower than the theoretical strength of an ideal crystal.
Crystal defects or imperfections could be of three types—
point defects
line defects
area defects.
3. What is the difference between slip and twin?
A.
4. Explain working principle of ultra-sonic testing.
A. Ultrasonic inspection is a non-destructive method in which beams of high-frequency sound waves are introduced into the material being inspected. The sound waves travel through the material with some attendant loss of energy and are reflected at interfaces. The reflected beam is detected and analyzed to define the presence and location of flaws.
Ultrasonic inspection employs ultrasonic waves. These waves are mechanical waves (in contrast to light or x-rays) that consist of oscillations or vibrations of the atomic or molecular particles of a substance. Ultrasonic wave behave essentially in the same way as audible sound waves. Most ultrasonic inspection is done at frequencies between 1 to 25 megacycles, well above the audible or sonic range, which is about 20 cycles to 20 kilo- cycles per second The directivity of ultrasonic vibrations increases with an increase in their frequency. At a frequency, of the order of one megacycle per second, the angle of divergence is so small that they may be called ‘ultrasonic beams".
5. Explain working principle of Radiography testing.
A.RADIOGRAPHIC INSPECTION :
Radiography is based on the fact that when highly penetrating rays, such as x-rays, are passed through a metal object, they are partly absorbed by the metal. The degree of absorption of the x-rays through the metal varies, depending upon the lack of homogeneity of material in different areas in. the metal. Areas having defects, such as blowholes and cracks, metal would absorb less x-rays than the remaining areas. This varying degree of absorption of x-rays can be determined by placing a sensitive photographic film on the other side of the specimen.
The photographic film. is darkened differently at different places depending upon the position of the defects in the metal. Regions more penetrable to x-rays radiation will be darker and the areas of high absorption will be lighter. Production of such a radiograph is shown in Fig. 16.3.
The penetration power of x-rays depends upon the wavelength of the
rays. which mainly varies from 10 to 0.1 Angstrom. The lower the wave-
length. the more penetrating the rays. Generally, these x-rays can pass through steel specimens up to 100 mm thick, copper and its alloys up to 60 mm thick and aluminium and its alloys up to 400 mm thick.

3 marks Questions:
6. Explain the Bragg’s law of X-ray diffraction in detail with neat sketch.
A.
• The crystal an diffract x ray because the inter planner spacing in crystal lattice is of some order as that of wavelength of x ray.
• Diffraction: It is a bending of line around the corner and it depend on wavelength.
• Diffraction due to inter planner spacing.
• separation of layer= x RAY wavelength.
• X-ray transmitted to words the crystal lattice. And this Xray are reflected and detected by detector
• Ray 2 travel more distance then ray one.
• Extra difference is DE+EF then if ray 2 and ray 1 have a same of phase after reflection thus path difference is decide if both are in same phase or not.
The different layers of crystal
7. What are the basic processing steps of a liquid penetrant inspection?
A. Steps of Liquid Penetrant Testing : The general steps can be summarized as follows:
1. Surface Preparation : ). One of the most critical steps of a liquid a person with a penetrant testing is the surface preparation. The surface must be free of oil, grease, water, or other contaminants that may prevent Penetrant from entering flaws. The sample may also require etching if mechanical operations such as machining, sanding, or grit blasting have been performed. These and other mechanical operations can smear metal over the flaw opening and prevent the penetrant from entering.
2. Penetrant Application : penetrant material Once the surface has been thoroughly cleaned and dried, the is applied by spraying, brushing, or immersing the part in a penetrant bath.
3. Penetrant Dwell : The penetrant is left on the surface for a sufficient time allow as much penetrant as possible to be drawn from or to seep into a defect. Penetrant dwell time is the total with the times are by to time that the penetrant is in contact part surface. usually the required being dwell from five Dwell recommended penetrant producers or by the specification followed. Minimum times typically range to 60 minutes. 4. Excess Penetrant Removal : This is the most delicate part of the inspection procedure because the excess penetrant must be removed from the surface of the sample while removing as little penetrant as possible from defects. Depending on the penetrant system used, this step may involve cleaning with a solvent, direct rinsing with water, or first treating the part with an emulsifier and then rinsing with water
5. Developer Application : A thin layer of developer is then applied to the sample to penetrant trapped in flaws back to the surface where it will be visible. draw Developers come in a variety of forms that may be applied by dusting ( ( dry powders wet developers ). ), dipping, or spraying
6. Indication Development : period The developer Photo Courtesy of Contoso is allowed to stand on the part surface for a of time sufficient to permit the extraction of the trapped penetrant out of any surface flaws. This development time is usually a minimum of 10 minutes. Significantly longer times may be necessary for tight cracks.
7. Inspection : Inspection is then performed from any flaws which may be present.
8. Clean Surface : under appropriate lighting The final step in the process is to thoroughly clean the part surface to remove the developer from the parts that were found to be acceptable. to detect indications
8. Differentiate between substitutional and interstitial solid solution.
A.
Substitutional solid solution
|
Interstitial solid solution
|
Substitutional Solid Solution is formed when some of the atoms of solvent are replaced by the solute atoms at their normal lattice points, as shown in Fig.. In the formation of substitutional solid solutions, an element A cannnot dissolve any amount of element B, its limit (known as solid solubility limit) is determined by certain factors. These factors were first studied by Hume Rothery and are
known as Hume Rothery Rules.
|
interstitial solid solution is formed when
atoms of small atomic radii fit into the empty spaces or interstices of the lattice structure of the solvent atoms as shown in Fig.. Since the empty spaces of the lattice structure are limited in size, only atoms with atomic radii less than I angstrom are likely to form interstitial solid solu-tions.
Interstitial solid solutiom normally have limited solid solubility. The
well known example of this group is interstitial solid solution of carbon in iron. y-iron can dissolve uptu 2 per cent carbon at 1147 'C. This interstitial solid solution Of carbon in iron is the basis for hardening in steel.
Interstitial solid solution ot hydrogen in min formed during acid pickling (cleaning), plating or welding operations With steel causes a sharp decrease in ductility of steel. This harmful phenomenon is known as hydrogen embritllemem.
|
9. Write difference between crystalline solid and non-crystalline solid.
A. Crystalline Solids: Crystalline Solids have an evenly distributed three-dimensional arrangement of atoms, ions, or molecules.
Non-crystalline solids: Non-crystalline solids do not have a consistent arrangement of particles.
Properties of Crystalline and Non-crystalline Solids
Geometrical Shape
Crystalline Solids: Crystalline solids have a well-defined geometrical shape due to the regular arrangement of unit cells.
Non-crystalline Solids: Non-crystalline solids do not have well-defined geometrical shape.
Range Order
Crystalline Solids: Crystalline solids have a long range order.
Non-crystalline Solids: Non-crystalline solids have a short range order.
Melting Point
Crystalline Solids: Crystalline solids have a definite melting point.
Non-crystalline Solids: Non-crystalline solids melt over a range.
Heat of Fusion
Crystalline Solids: Crystalline solids have a high fixed value for the heat of fusion.
Non-crystalline Solids: Non-crystalline solids do not have a fixed value for the heat of fusion.
Properties of Solids
Crystalline Solids: Crystalline solids are true solids. They show all the properties of solids.
Non-crystalline Solids: Non-crystalline solids do not show all the properties of solids. Therefore, they are called “pseudo solids”.
Energy
Crystalline Solids: Energy in crystalline solids is lower than that of non-crystalline solids.
Non-crystalline Solids: Nature favours crystalline solids due to the low energy arrangement.
10. State different types of unit cells and sketch their geometries.
A.• The four basic types of unit cells are :
Simple
Body Centred cubic
Face centred cubic
Base centred cubic
Hexagonal Closed packed
11. What is meant by ‘ Bravai’s Lattice ’? Write the names of seven crystal system.
A. In geometry and crystallography, a Bravai’s lattice is an infinite array of discrete points in three dimensional space generated by a set of discrete translation operations described by:
When the discrete points are atoms, ions, or polymer strings of solid matter, the Bravais lattice concept is used to formally define a crystalline arrangement and its (finite) frontiers. A crystal is made up of a periodic arrangement of one or more atoms (the basis) repeated at each lattice point. Consequently, the crystal looks the same when viewed from any equivalent lattice point, namely those separated by the translation of one unit cell (the motif).
12. Define space lattice. What are the characteristics of a space Lattice?
A. A crystalline substance is one which is made of crystals or parts of crystals. In a crystal, the atoms are arranged in a periodic and regular geometric pattern in space. The arrangement of atoms in a crystal can be described with respect to three dimensional net of straight lines, called a space Lattice.
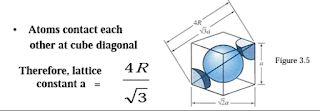
13. Explain with neat sketches the arrangement of atoms in B.C.C,
F.C.C and H.C.P Lattice.
A.
BCC
FCC
HCP
14. Explain simple cubic crystal structure with neat sketch.
A.
15. Draw the cubic crystal planes for following Miller indices:
(i) (0 1 0) (ii) (0 0 1) (iii) (1 2 0).
A.




















0 comments:
Post a Comment